Abstract
Background:
In China, 90% of boys with severe hemophilia A (SHA) manifest significant arthropathy by age 6 years (Wu R et al Haemophilia, 2014). Short‐term pilot studies demonstrated that low‐dose prophylaxis (10‐15 IU/kg x2 /week) reduced joint bleeds by 80% but was ineffective in eliminating joint damage (Wu R et al Haemophilia, 2011, Tang L et al Haemophilia, 2013). Optimizing prophylaxis in China for boys with SHA is an urgent priority.
Aims:
To investigate the efficacy of an individualized, dose escalating prophylaxis protocol in young boys with SHA in China
Methods:
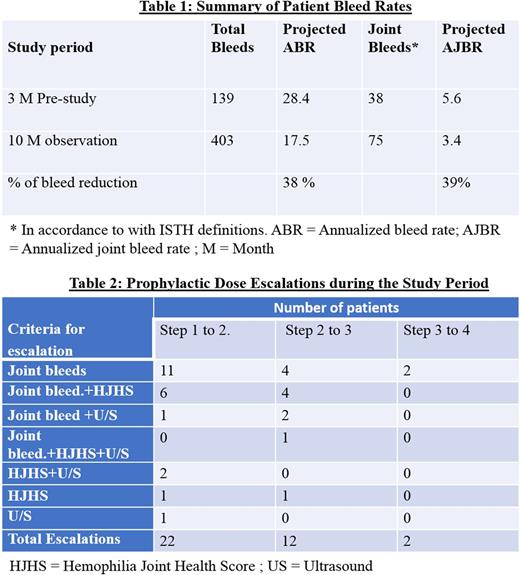
This is a prospective, ethics approved clinical trial conducted at 2 major Hemophilia Treatment centers in China. Boys with SHA (baseline FVIII < 2%), ages 1‐7 years, inhibitor negative, >50 Exposure Days and a history of index joint (elbows, knees, ankles) bleeds were eligible. The design included 4 dose escalation steps; Step 1: 10‐15 IU/kg x2/week; Step 2: 10‐15 IU/Kg, x3/week; Step 3: 15‐20 IU/Kg x3/week; and Step 4: 20‐25 IU/Kg every other day. A priori escalation criteria were based on the frequency of index joint bleeds, scores from the Hemophilia Joint Health Score (HJHS) and Ultrasound (US) examinations of index joints.
Results:
30 patients were consented and enrolled, with only 1 drop-out. The mean age was 4.8 yrs. (range: 2.1 ‐ 7.9 yrs.) and the median follow up observation period was 10 months (range: 6 ‐11 months). In total, the patients have completed 76% of the required number of months on study (275/360 patient months). Musculoskeletal (MSK) assessment showed a total of 19 boys with target joints (65%). In addition, a total of 22 patients completed 36 dose escalations. Of these patients currently on prophylaxis, 8 patients are on step 1 (28%); 9 on step 2 (31%), 10 on step 3 (34%); and 2 on step 4 (7%). The criterion for escalations are summarized in Table 2.
Conclusions:
This is the first prospective, individualized, dose escalating prophylaxis study for boys with SHA in China with the goal of minimizing bleeding and joint damage. Findings from the HJHS and US examinations to guide dose escalation, in conjunction with index joint bleeds, is a unique feature of this clinical trial. Our results indicate a promising reduction in joint bleeds by using the 4-step escalation low dose regimens with equal utilization of Steps 1, 2 and 3 by the patients. Low dose prophylaxis may be ineffective in some cases and assessments of bleeding patterns and joint structure/function is important to inform modification of prophylactic regimens. These observations will be useful in the design of cost effective prophylaxis regimens for boys with SHA in China.
This study is financially supported by a grant from Bayer HealthCare Co. Ltd., China.
Wu: Bayer Healthcare Co. Ltd., China: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal